Technology
|
DME Production Technology DME (CH3OCH3) is synthesized from synthesis gas (H2,CO), which is obtained from such fossil resources as natural gas and coal as well as renewable resources, for example biomass. There are two kinds of production process routes: Direct method (One step process) which directly synthesizes DME from synthesis gas and Indirect method (Two step process) which synthesizes methanol (CH3OH) as the first step and produces DME by dehydration of methanol as the second step. In case of Indirect method, methanol synthesis step affects substantially the whole process efficiency.
・Two step process (Indirect DME Synthesis: Methanol synthesis +Dehydration) (1) Methanol synthesis: 4H2+2CO →2CH3OH (2) Dehydration of Methanol: 2CH3OH →CH3OCH3+H2O
・One step process (Direct DME Synthesis) In addition to the above reactions (1) and (2), (3) Water gas shift reaction is promoted in One Single reactor. (3) Water gas Shift reaction: H2O+CO → H2+CO2 (4) DME synthesis: 3H2+3CO→CH3OCH3+CO2
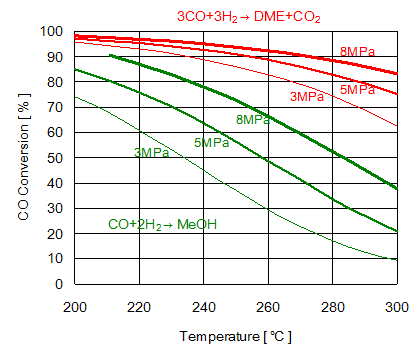
Both methanol synthesis reaction and DME synthesis reaction are theoretically controlled by chemical equilibrium changing with reaction temperature and pressure. Catalyst system activating reaction plays an important role in the actual reactor. Figure 2 compares equilibrium conversion of methanol synthesis reaction with that of DME synthesis reaction. DME synthesis is expected to have higher conversion at lower reaction pressure. As both reactions are exothermic and catalyst loses its activity at high temperature, control of reaction temperature is significant.
|
| « prev | top | next » |
 Japanese
Japanese English |
English |